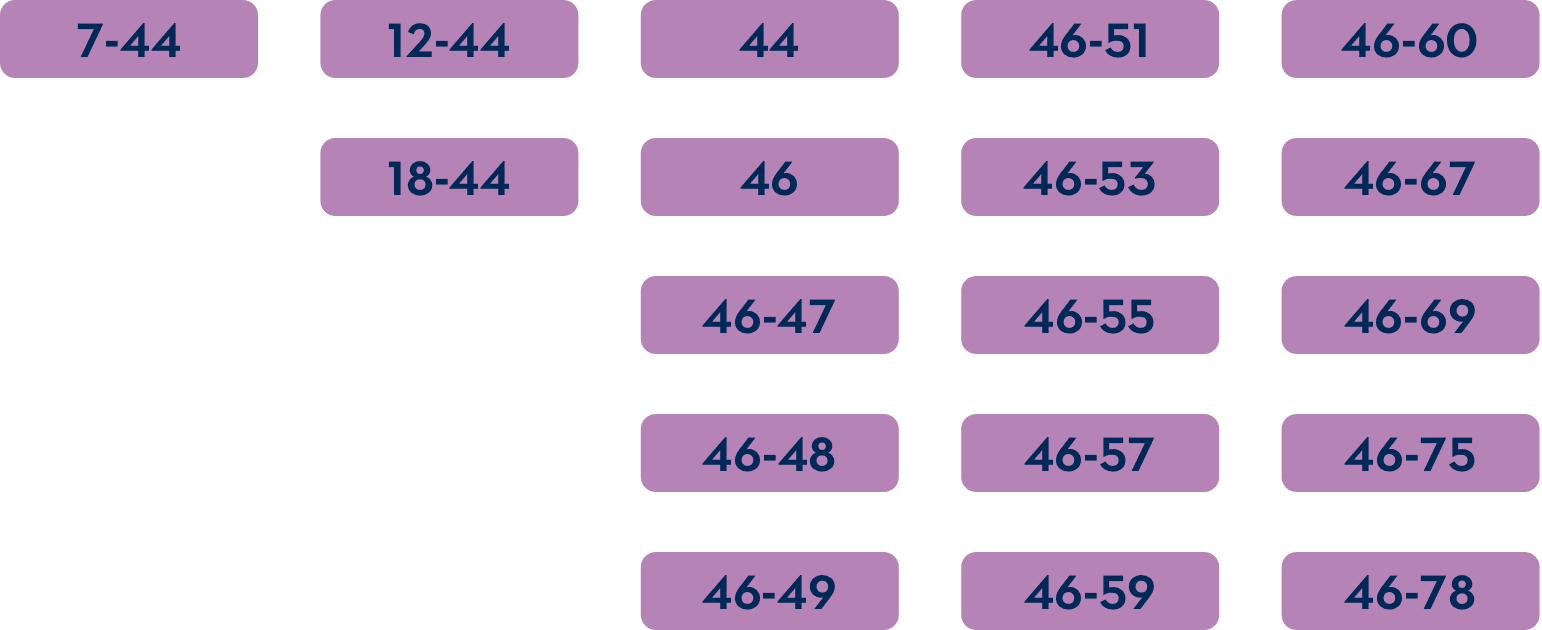
Which mutations are amenable to exon 45 skipping?1-3
For exon skipping to work, exon 45 must be present in the patient's DMD gene.
The common DMD deletions that are theoretically amenable to exon 45 skipping include:
IMPORTANT SAFETY INFORMATION
AMONDYS 45 is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping. This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with AMONDYS 45. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
IMPORTANT SAFETY INFORMATION
CONTRAINDICATIONS:
AMONDYS 45 is contraindicated in patients with a known serious hypersensitivity to casimersen or any of the inactive ingredients in AMONDYS 45. Instances of hypersensitivity including angioedema and anaphylaxis have occurred.
WARNINGS AND PRECAUTIONS
Hypersensitivity Reactions:
Hypersensitivity reactions, including angioedema and anaphylaxis, have occurred in patients who were treated with AMONDYS 45. If a hypersensitivity reaction occurs, institute appropriate medical treatment, and consider slowing the infusion, interrupting, or discontinuing the AMONDYS 45 infusion and monitor until the condition resolves. AMONDYS 45 is contraindicated in patients with known serious hypersensitivity to casimersen or to any of the inactive ingredients in AMONDYS 45.
Kidney Toxicity:
Kidney toxicity was observed in animals who received casimersen. Although kidney toxicity was not observed in the clinical studies with AMONDYS 45, kidney toxicity, including potentially fatal glomerulonephritis, has been observed after administration of some antisense oligonucleotides. Kidney function should be monitored in patients taking AMONDYS 45. Because of the effect of reduced skeletal muscle mass on creatinine measurements, creatinine may not be a reliable measure of kidney function in DMD patients. Serum cystatin C, urine dipstick, and urine protein-to-creatinine ratio should be measured before starting AMONDYS 45. Consider also measuring glomerular filtration rate using an exogenous filtration marker before starting AMONDYS 45. During treatment, monitor urine dipstick every month, and serum cystatin C and urine protein-to-creatinine ratio (UPCR) every three months. Only urine expected to be free of excreted AMONDYS 45 should be used for monitoring of urine protein. Urine obtained on the day of AMONDYS 45 infusion prior to the infusion, or urine obtained at least 48 hours after the most recent infusion, may be used. Alternatively, use a laboratory test that does not use the reagent pyrogallol red, as this reagent has the potential to cross react with any AMONDYS 45 that is excreted in the urine and thus lead to a false positive result for urine protein.
If a persistent increase in serum cystatin C or proteinuria is detected, refer to a pediatric nephrologist for further evaluation.
ADVERSE REACTIONS:
Adverse reactions occurring in at least 20% of patients treated with AMONDYS 45 and at least 5% more frequently than in the placebo group were (AMONDYS 45, placebo): upper respiratory infections (65%, 55%), cough (33%, 26%), pyrexia (33%, 23%), headache (32%, 19%), arthralgia (21%, 10%), and oropharyngeal pain (21%, 7%).
Other adverse reactions that occurred in at least 10% of patients treated with AMONDYS 45 and at least 5% more frequently than in the placebo group were: ear pain, nausea, ear infection, post-traumatic pain, and dizziness and light-headedness.
Other adverse events may occur.
To report SUSPECTED ADVERSE REACTIONS, contact Sarepta Therapeutics, Inc. at 1-888-SAREPTA (1-888-727-3782) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Before administration, please see the full Prescribing Information for AMONDYS 45 (casimersen).
INDICATIONS AND USAGE
AMONDYS 45 is indicated for the treatment of Duchenne muscular dystrophy (DMD) in patients who have a confirmed mutation of the DMD gene that is amenable to exon 45 skipping. This indication is approved under accelerated approval based on an increase in dystrophin production in skeletal muscle observed in patients treated with AMONDYS 45. Continued approval for this indication may be contingent upon verification of a clinical benefit in confirmatory trials.
References: 1. AMONDYS 45 [package insert]. Cambridge, MA: Sarepta Therapeutics, Inc. 2024. 2. Aartsma-Rus A, Van Deutekom JCT, Fokkema IF, et al. Entries in the Leiden Duchenne muscular dystrophy mutation database: an overview of mutation types and paradoxical cases that confirm the reading-frame rule. Muscle Nerve. 2006;34(2):135-144. 3. Wood MJA, Gait MJ, Yin H. RNA-targeted splice-correction therapy for neuromuscular disease. Brain. 2010;133(pt 4):957-972. 4. Ciafaloni E, Fox DJ, Pandya S, et al. Delayed diagnosis in Duchenne muscular dystrophy: data from the Muscular Dystrophy Surveillance, Tracking, and Research Network (MD STARnet). J Pediatr. 2009;155(3):380-385. 5. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 1: diagnosis, and neuromuscular, rehabilitation, endocrine, and gastrointestinal and nutritional management. Lancet Neurol. 2018;17(3):251-267. 6. Cowen L, Mancini M, Martin A, et al. Variability and trends in corticosteroid use by male United States participants with Duchenne muscular dystrophy in the Duchenne Registry. BMC Neurol. 2019;19(1):84. 7. Birnkrant DJ, Bushby K, Bann CM, et al. Diagnosis and management of Duchenne muscular dystrophy, part 2: respiratory, cardiac, bone health, and orthopaedic management. Lancet Neurol. 2018;17(4):347-361. 8. Raman SV, Hor KN, Mazur W, et al. Stabilization of early Duchenne cardiomyopathy with aldosterone inhibition: results of the multicenter AIDMD trial. J Am Heart Assoc. 2019;8(19):e013501. 9. McDonald CM, Abresch RT, Carter GT, et al. Profiles of neuromuscular diseases: Duchenne muscular dystrophy. Am J Phys Med Rehabil. 1995;74(suppl 5):S70-S92. 10. Houwen-van Opstal SLS, Heutinck L, Jansen M, et al. Occurrence of symptoms in different stages of Duchenne muscular dystrophy and their impact on social participation. Muscle Nerve. 2021;64(6):701-709. 11. Pane M, Coratti G, Brogna C, et al. Longitudinal analysis of PUL 2.0 domains in ambulant and non-ambulant Duchenne muscular dystrophy patients: how do they change in relation to functional ability? J Neuromuscul Dis. 2023;10(4):567-574. 12. McDonald CM, Gordish-Dressman H, Henricson EK, et al; CINRG investigators for PubMed. Longitudinal pulmonary function testing outcome measures in Duchenne muscular dystrophy: long-term natural history with and without glucocorticoids. Neuromuscul Disord. 2018;28(11):897-909. 13. Power A, Poonja S, Disler D, et al. Echocardiographic image quality deteriorates with age in children and young adults with Duchenne muscular dystrophy. Front Cardiovasc Med. 2017;4:82. 14. Data on file. Sarepta Therapeutics, Inc.
This information is intended for U.S. healthcare professionals only.
